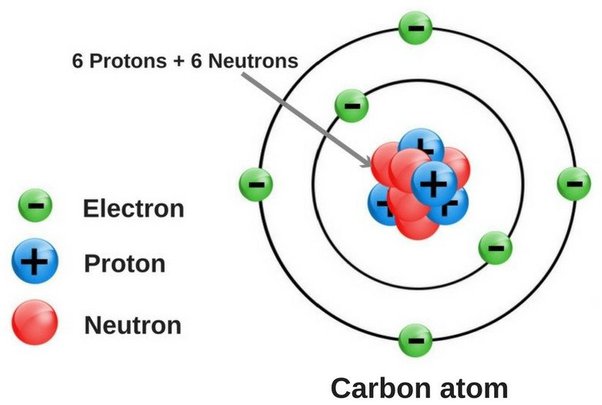
Oxygen has 8 electrons. This element has 8 protons and 8 electrons in its atomic structure.
Oxygen is a crucial element found in the Earth’s atmosphere, making up around 21% of the air we breathe. With its atomic number of 8, oxygen possesses 8 electrons that play a significant role in various chemical reactions and bonding processes.
Its ability to form stable compounds and support life processes makes oxygen essential for living organisms. Understanding the electron configuration of oxygen is vital in comprehending its reactivity and importance in sustaining life on our planet. Let’s delve deeper into the fascinating world of oxygen and explore its electron arrangement in greater detail.
Introduction To Oxygen
Oxygen is one of the most essential elements found on Earth. It plays a crucial role in supporting life and is vital for various biological processes. Understanding the elemental basics of oxygen and its role in nature is key to comprehending its significance in our everyday lives.
The Elemental Basics
Oxygen, with the chemical symbol O and atomic number 8, is part of the chalcogen group on the periodic table. It is a highly reactive nonmetal that readily forms compounds with other elements. Oxygen is the third most abundant element in the universe, following hydrogen and helium.
In its natural state, oxygen exists as a diatomic molecule, meaning it consists of two oxygen atoms bonded together (O2). This molecular form is essential for sustaining life, as it is the form in which oxygen is commonly found in the Earth’s atmosphere.
Oxygen’s Role In Nature
Oxygen is integral to the processes of respiration and combustion, making it indispensable for supporting life as we know it. In the natural world, oxygen is produced through photosynthesis, a process carried out by plants, algae, and some bacteria. During photosynthesis, these organisms convert carbon dioxide and sunlight into oxygen and glucose.
Oxygen’s role extends beyond the production of energy. It is crucial for the survival of various organisms, including humans, as it is required for cellular respiration. Through respiration, oxygen is utilized by cells to generate energy from glucose, enabling the proper functioning of bodily systems.
Additionally, oxygen plays a vital role in the Earth’s ozone layer, which shields the planet from harmful ultraviolet radiation. The ozone layer is formed by the interaction of oxygen molecules (O2) with ultraviolet light, creating ozone (O3). This protective layer acts as a natural barrier, preventing excessive UV radiation from reaching the Earth’s surface.
In conclusion, understanding the elemental basics of oxygen and its role in nature is essential for appreciating its significance. From supporting life through respiration to contributing to the Earth’s ozone layer, oxygen is a fundamental element that impacts our daily existence in numerous ways.
Credit: socratic.org
Diving Into The Atomic Structure
Atoms are the building blocks of matter, consisting of a nucleus made up of protons and neutrons, and a cloud of negatively charged electrons that orbit the nucleus. Oxygen, with the atomic number 8, has 8 protons and 8 electrons in its neutral state. Let’s dive deeper into the atomic structure of oxygen and explore how many electrons it has.
Atoms And Electrons
Electrons are negatively charged particles that are located outside the nucleus in specific energy levels or shells. These shells determine how many electrons an atom can hold and the order in which they are filled. Oxygen has 2 electrons in its innermost shell and 6 electrons in its outermost shell, which means it can hold up to 8 electrons in total.
Oxygen’s Place On The Periodic Table
How Many Electrons Does Oxygen Have? Oxygen is a member of the chalcogen group on the periodic table, located in group 16. It is a nonmetal and is highly reactive due to its electronegativity, which means it has a strong tendency to attract electrons towards itself. This property makes oxygen a key element in many chemical reactions and essential for life on Earth.
Understanding the atomic structure of oxygen and the number of electrons it has is crucial in understanding its chemical and physical properties. With 8 electrons in its neutral state, oxygen is a highly reactive nonmetal that plays a vital role in the world around us.
Counting Oxygen’s Electrons
Oxygen contains 8 electrons in its atomic structure, with 2 electrons in its inner shell and 6 in its outer shell. This configuration gives oxygen the ability to form stable compounds with other elements.
Oxygen, an essential element for life, plays a crucial role in various chemical reactions. To understand its behavior and interactions, it is important to know the number of electrons present in an oxygen atom. By counting the electrons, we can gain valuable insights into the atomic structure and properties of oxygen.
Atomic Number Insight
The atomic number of an element corresponds to the number of protons in its nucleus. For oxygen, the atomic number is 8. This means that an oxygen atom contains 8 protons, which also defines its unique chemical identity. In a neutral oxygen atom, the number of electrons is equal to the number of protons, so oxygen typically has 8 electrons.
Electron Configuration
The arrangement of electrons within an atom is known as its electron configuration. Oxygen follows the electron configuration pattern of 1s2 2s2 2p4. This indicates that the first energy level (1s) contains 2 electrons, the second energy level (2s) contains 2 electrons, and the second energy level (2p) contains 4 electrons.
To visualize the electron configuration, we can represent it in a table format:
| Energy Level | Sublevel | Number of Electrons |
|---|---|---|
| 1 | s | 2 |
| 2 | s | 2 |
| 2 | p | 4 |
From the table, we can see that oxygen’s electrons are distributed across the first and second energy levels, with the second energy level having two sublevels: s and p. The electron configuration provides a roadmap for understanding the chemical behavior and reactivity of oxygen.
Knowing the electron count of oxygen is vital in fields such as chemistry, biology, and physics. It helps scientists predict and explain the behavior of oxygen in different chemical reactions, its ability to form bonds, and its involvement in various biological processes.
In conclusion, counting oxygen’s electrons reveals that it typically possesses 8 electrons. This information, along with its atomic number and electron configuration, is fundamental in understanding the characteristics and behavior of this essential element.
Chemical Bonding And Valency
Oxygen has 8 electrons in total, with 6 in its valence shell. This makes it capable of forming two covalent bonds.
Chemical bonding is the process by which atoms combine to form molecules or compounds. It plays a crucial role in determining the properties and behavior of substances. Understanding chemical bonding helps us comprehend how elements interact with each other to create various compounds.
Covalent Bonding Explained
In covalent bonding, atoms share electrons to achieve a stable electron configuration. This type of bonding commonly occurs between nonmetal elements. Covalent bonds are formed when atoms have a similar electronegativity, meaning they have an equal tendency to attract shared electrons.
Covalent bonds can be represented using Lewis dot structures, where valence electrons are depicted as dots surrounding the atomic symbol. These structures help visualize the sharing of electrons between atoms.
Oxygen’s Valence Electrons
Oxygen, with an atomic number of 8, has six valence electrons. Valence electrons are the electrons in the outermost energy level of an atom. For oxygen, these electrons are located in the 2p orbital.
The electron configuration of oxygen is 1s2 2s2 2p4. The 2s orbital is fully occupied with two electrons, while the 2p orbital has four electrons. These four valence electrons in the 2p orbital are available for bonding with other atoms.
Oxygen’s valence electrons enable it to form covalent bonds with other elements. By sharing its two unpaired electrons, oxygen can complete its valence shell and achieve a stable electron configuration, similar to that of a noble gas.
In covalent compounds, oxygen commonly forms double bonds, sharing two electrons with another atom. This is evident in molecules such as carbon dioxide (CO2), where oxygen forms two double bonds with carbon.
Oxygen’s ability to form multiple covalent bonds makes it a vital element in many organic and inorganic compounds. Its valence electrons and covalent bonding characteristics contribute to its wide range of chemical and biological roles.
Isotopes Of Oxygen
The isotopes of oxygen refer to the different forms of oxygen that exist with varying numbers of neutrons in their nuclei. Oxygen has three primary isotopes: oxygen-16, oxygen-17, and oxygen-18. These isotopes have different numbers of neutrons, resulting in variations in their atomic masses and properties.
Variations In Oxygen Isotopes
Oxygen-16 is the most abundant isotope, constituting approximately 99.76% of natural oxygen. Oxygen-18 is present in about 0.2% and oxygen-17 in trace amounts. The variations in isotopic composition have significant implications in diverse fields, including geology, climatology, and biology.
Electron Counts In Isotopes
Each isotope of oxygen possesses a distinct number of electrons. Oxygen-16 has 8 protons and 8 neutrons, resulting in 8 electrons. Oxygen-17 and oxygen-18 have different numbers of neutrons, leading to variations in their electron counts. These differences in electron counts contribute to the unique chemical behaviors of the oxygen isotopes.
Oxygen Ions: Gaining And Losing Electrons
Oxygen, a key element in the Earth’s atmosphere, plays a crucial role in various chemical reactions. When oxygen gains or loses electrons, it forms ions with distinct properties and functions. Let’s delve into the fascinating world of oxygen ions and their electron behavior.
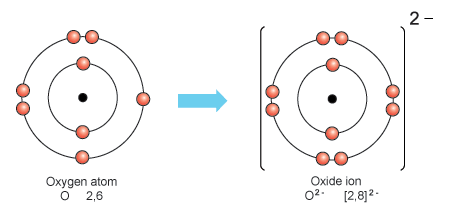
Oxide And Peroxide Ions
Oxide ions are formed when oxygen gains two electrons, resulting in a charge of -2. These ions are commonly found in compounds such as metal oxides and are crucial in various industrial processes. Conversely, peroxide ions form when oxygen gains one additional electron, yielding a charge of -1. These ions are present in compounds like hydrogen peroxide, known for their antiseptic and bleaching properties.
The Role Of Electrons In Ion Formation
Electrons play a pivotal role in the formation of oxygen ions. When oxygen gains or loses electrons, its chemical properties and reactivity are significantly altered. The transfer of electrons leads to the creation of charged particles with distinct characteristics, influencing their behavior in chemical reactions.
Quantum Mechanics And Electron Shells
Oxygen has 8 electrons, with 2 in the first shell and 6 in the second shell according to quantum mechanics. This arrangement allows oxygen to form stable chemical bonds, making it essential for various biological and industrial processes.
Energy Levels In Oxygen
Oxygen has 8 electrons distributed across its energy levels.
Quantum Numbers And Electron Positions
Electrons in oxygen occupy specific quantum numbers. Quantum mechanics governs electron distribution in oxygen. Each electron shell can hold a specific number of electrons. Electrons are positioned based on quantum numbers in oxygen.
Credit: www.quora.com
Practical Applications
Oxygen has 8 electrons. This knowledge is essential in various practical applications, including chemistry, physics, and biology. Understanding the electron configuration of oxygen is crucial for comprehending its behavior in different chemical reactions and molecular interactions.
Oxygen In Industrial Processes
Oxygen is used in various industrial processes like steel production and waste treatment.
Understanding Electron Transfer In Reactions
Electron transfer involving oxygen is crucial in chemical reactions. – Oxygen’s role in oxidation reactions is fundamental. – Oxygen’s electron configuration influences its reactivity. – Oxygen molecules accept electrons in reactions. In industries, oxygen’s electron behavior affects processes significantly.
Frequently Asked Questions
How Many Electrons Does An Oxygen Atom Have?
Oxygen has 8 electrons, arranged in energy levels or shells. The first shell contains 2 electrons, while the second shell contains 6 electrons. This configuration gives oxygen a stable structure and allows it to form bonds with other elements.
Why Does Oxygen Need 8 Electrons?
Oxygen requires 8 electrons to achieve a stable octet configuration, following the octet rule. This stable configuration allows oxygen to attain a lower energy state and become more chemically stable. It enables oxygen to form various compounds and participate in chemical reactions.
How Do Oxygen’s Electrons Contribute To Its Reactivity?
The 6 valence electrons in oxygen’s outer shell make it highly reactive. Oxygen can gain, lose, or share electrons to complete its outer shell, forming compounds with other elements. This reactivity underpins its role in supporting combustion, respiration, and the formation of diverse organic and inorganic compounds.
Conclusion
Oxygen has eight electrons, with two in its innermost shell and six in its outermost shell. These electrons are crucial for oxygen’s chemical properties, including its ability to react with other elements to form compounds. Understanding the number and arrangement of electrons in oxygen is fundamental to understanding its behavior in various contexts, from atmospheric chemistry to biological processes.
Overall, knowing the electron configuration of oxygen is essential for a wide range of scientific fields, making it a fascinating and important element to study.